Beginning in 2009, Ventana BioSciences will offer biologics manufacturing services under GMP in a State of California Food and Drug Bureau licensed facility.
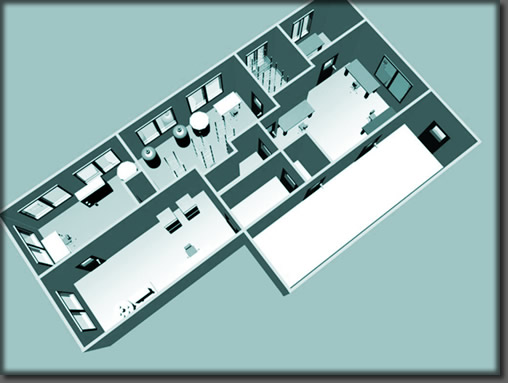
The facility consists of:
1000 ft2 clean-rooms
(100,000 and 10,000 with Class 100 BSC's)
500 ft2 controlled materials storage area
Validated Laminar Flow Hoods, Liquid Nitrogen Freezers and CO2 incubators
The following Quality Systems are already in place:
- Document control
- Facitlity control and environmental monitoring
- Materials control
Home | About Us | Viral Vectors | Mammalian Cell Expresion | Mammalian Cell Culture/Cell Banking
Process Development | Anaylitical | YourLab | Clinical Production | References/Knowledge Base | Contact Us
![]()
Phone: 858-405-3506
